bch 108 Assignment Question and Answers 2025
BCH 108 Assessment Manual Solution




ANSWERS
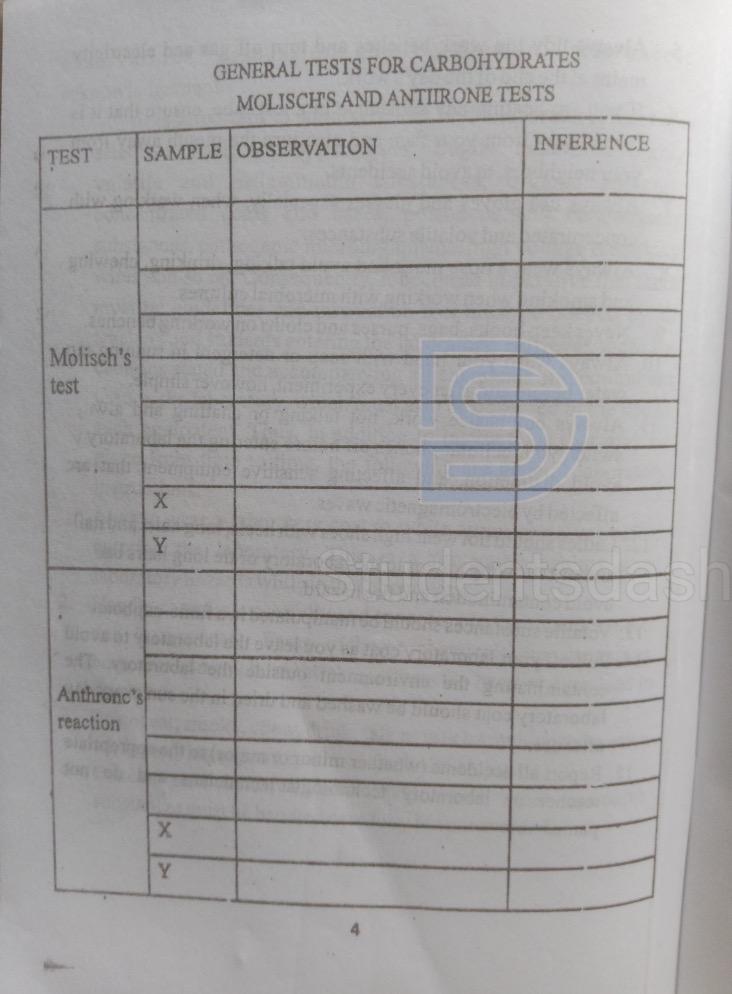
Page 4.
Answers loading….
Page 5
1. Why do sugars give a positive Molisch’s test?
Sugars give a positive Molisch’s test because, in the presence of concentrated sulphuric acid, they are dehydrated to form furfural (from pentoses) or hydroxymethylfurfural (from hexoses). These furfural derivatives then react with α-naphthol to produce a purple or violet-colored complex, indicating the presence of carbohydrates.
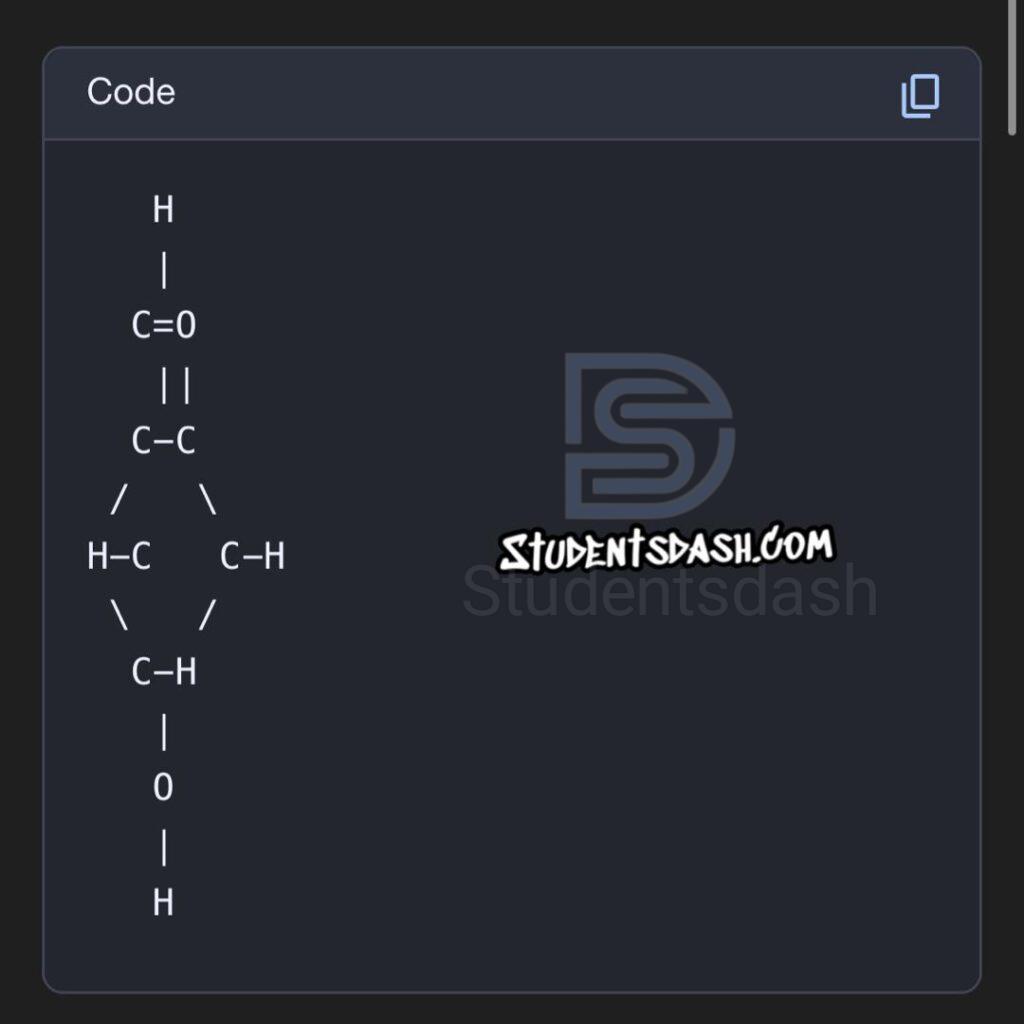
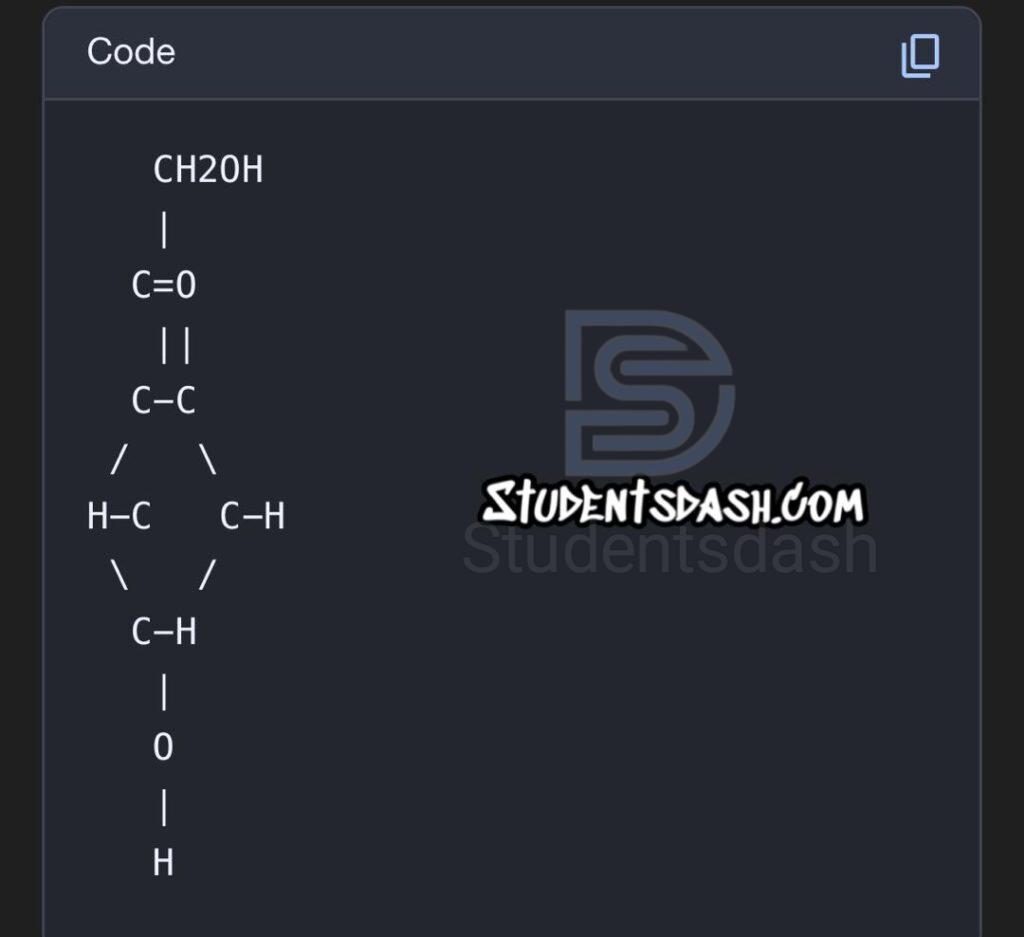
2. State and draw the structure of the dehydration product of furanoses and pyranoses in Molisch’s test.
- From pentoses (furanoses) → dehydration produces furfural.
- From hexoses (pyranoses) → dehydration produces 5-hydroxymethylfurfural.
Structures:
Furfural:
5-Hydroxymethylfurfural:
3. Explain the role of concentrated tetraoxosulphate (VI) acid in Molisch’s test.
Concentrated H₂SO₄ acts as a strong dehydrating agent. It removes water molecules from the carbohydrate, converting pentoses to furfural and hexoses to 5-hydroxymethylfurfural. It also provides the acidic environment required for the reaction with α-naphthol.
4. State the principle underlying the Molisch’s test.
Molisch’s test is based on the principle that all carbohydrates, when treated with concentrated sulphuric acid, undergo dehydration to yield furfural derivatives. These derivatives condense with α-naphthol to form a violet or purple-colored ring at the interface, indicating the presence of carbohydrates.
5. Describe the following terms, giving an example of each:
- Aldohexose – A hexose (six-carbon sugar) with an aldehyde functional group.
Example: Glucose (C₆H₁₂O₆). - Ketopentose – A pentose (five-carbon sugar) with a ketone functional group.
Example: Ribulose (C₅H₁₀O₅). - Aldotriose – A triose (three-carbon sugar) with an aldehyde functional group.
Example: Glyceraldehyde (C₃H₆O₃).

