CHM 101 (ICH 101) Past Question and Answers
Hey, as you may know the Ebonyi State University(Ebsu) year one exam is CBT BASED which means it will be written with computer, and today we will be drop only 11 Past Questions here to practice more use our STUDENTSDASH Practice code to Practice more on STUDENTSDASH APP.
In this article we CONTINUED the ICH 101 Past Question Paper and the possible solution, if the solutions are not yet complete please contact us on whatsapp.
ICH 101 Past Question
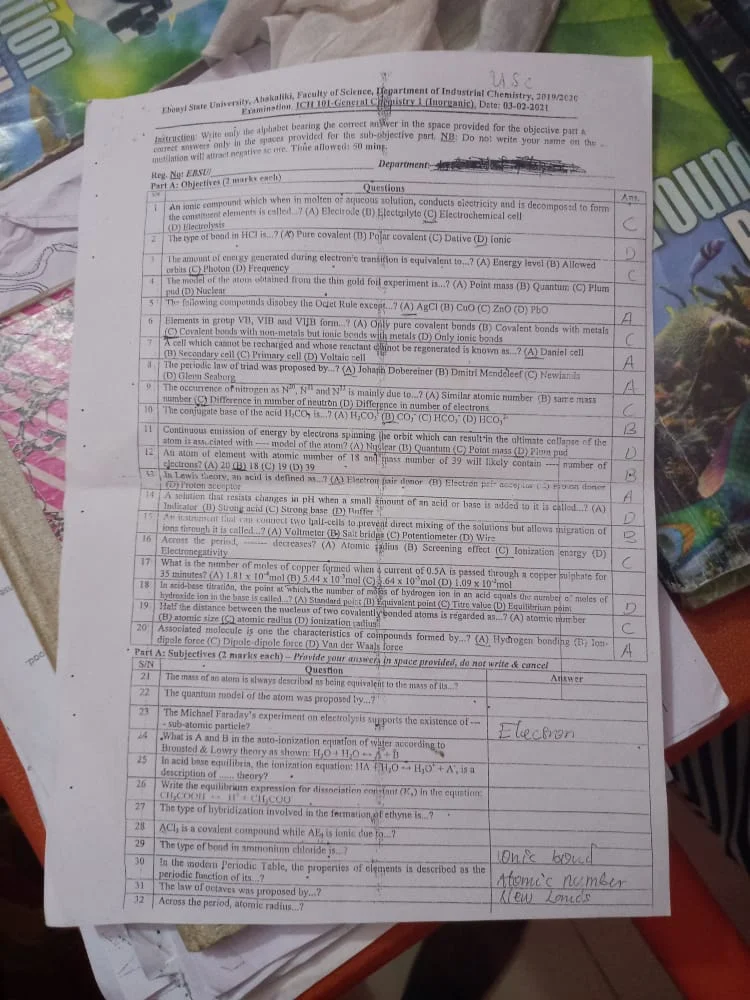
//1//An ionic compound which when in molten of aqueous solution, conducts electricity and is decomposed to form a constituent element is called ….?
//A//Electrode
//B//Electrolyte
//C//Electrochemical Cell
//D// Electrolysis
ans = B
//2// The type of bound in HCL is …..?
//A//Pure Covalent
//B//Porlar Covalent
//C//Dalive
//D//Ionic
ans = B
//3// The amount of energy generated during electron’s transition is equivalent to ….?
//A// Energy Level
//B//Allowed Orbits
//C//Photon
//D//Frequency
ans = C
//4// The model of the atoms obtained from the thin gold foil experiment is ….?
//A//Pint Mass
//B//Quantum
//C//Plumpud
//D//Nuclear
ans = D
//5//The following compounds disobeys the octet rule except …?
//A//AgCl
//B//CuO
//C//ZnO
//D//PbO
ans = A
//6// Elements in group VB, VIB and VIIB form ….?
//A// Only pure covalent bonds
//B//Covalent bonds with metal
//C//Covalent bonds with non-metal but ionic bonds with metals
//D//Only ionic bonds
ans = c
//7// A cell which cannot be recharged and whose rectant cannot be regenereted is known as ……?
//A// Daniel Cell
//B// Secondary Cell
//C//Primary Cell
//D//Voltalic Cell
ans = C
//8// The periodic law of triad was proposed by….?
//B// Dmitri Mendellef
//C//Newiands
//D//Gienn Seaborg
ans = A
//9// THe occurrence of nitrogen as N^20, N^21 and N^33 is mainly due to ….?
//A//Similar Atomic Number
//B//Samse Mass Number
//C//Difference in number of neutron
//D//Difference in number of electrons
ans = C
QUESTION NUMBER 10
The occurrence of nitrogen as N^20, N^21 and N^33 is mainly due to ….?
A. Similar Atomic Number
B. Samse Mass Number
C. Difference in number of neutron
D. Difference in number of electrons
ANSWER: C
QUESTION NUMBER 11
The conjugate base of the acid H2CO3 is….?
A. H2CO3
B. CO2
C. HCO2
D. HCO2^3
ANSWER: D
QUESTION NUMBER 12
The continous emission of energy vy electrons spinning the orbit which can result in the ultimate collapse of the atom is associated with—– model of the atom?
A. Nuclear
B. Quantum
C. Point Mass
D. Plum pud
ANSWER: D
QUESTION NUMBER 13
An atom of an element with atomic number of 18 and mass number of 39 will likely contain….. number of electrons.
A. 20
B. 18
C. 19
D. 39
ANSWER: C
QUESTION NUMBER 14
In lewis theory an acid is defined as …… ?
A. Electron pair donor
B. Electron pair acceptor
C. Neutron Donor
D. Proton acceptor
ANSWER: B
QUESTION NUMBER 15
A solution that resists changes in PH when a small amount of acid or base is added is known as….?
A. Indicator
B. Strong acid
C. Strong base
D. Buffer
ANSWER: D
QUESTION NUMBER 16
An instrument that can connect two half-cells to prevent direct mixing of the solutions but allows migration of ions through it is called?
A. Voltmeter
B. Salt Bridge
C. Potentiometer
D. Wire
ANSWER: B
QUESTION NUMBER 17
Across the period, —– decreases?
A. Atomic Radius
B. Screening Effect
C. Ionization Energy
D. Electronegativity
ANSWER: C
QUESTION NUMBER 18
What is the number of moles of copper formed when a current of 0.5A is passed through a copper sulphate for 35 minutes?
A. 1.8 X 10^-8mol
B. 5.44 x 10^-3mol
C. 5.64 x 10^-3 mol
D. 1.09 x 10^-3mol
ANSWER: C
QUESTION NUMBER 19
in acid base titration, the point at which the number of masses of hydrogen ion in an acid is equal the number of moles of hydroxide ion in the base is called….?
A. Standard Point
B. Equivalent Point
C. Titre Value
D. Equilibrium point
ANSWER: B
QUESTION NUMBER 20
Half the distance between the nucleus of two covalent bonded atoms is regarded as?
A. Atomic Number
B. Atomic Size
C. Atomic radius
D. Ionization Radius
ANSWER: C
QUESTION NUMBER 21
Associated Molecules is one of the characteristics of compounds formed by ….?
A. Hydrogen Bonding
B. Ion-dipole force
C. Dipule- dipole force
D. Van der Waals Force
ANSWER: A
QUESTION NUMBER 22
The mass of atom is always described as being equivalent to the mass of …?
A. The number of electrons in atomic unit
B. The number of protons in atomic unit
C. Number of neutrons in the atomic unit
D. Number of protons in the same unit
ANSWER: B
QUESTION NUMBER 23
The quantum mechanical model of an atom was proposed by ….?
A. Erwin Schrodinger
B. Godwin Rodinger
C. Emmynasouth
D. Niels Bohr
ANSWER: A
QUESTION NUMBER 24
The Michael Faraday’s experiment on electrolysis supports the existence of —– sub-atomic particle?
A. Electrons
B. Protons
C. Neutrons
D. No-atrons
ANSWER: A
QUESTION NUMBER 25
The type of hybridization involved in the fermation of ethyne is…?
A. Sp hybridised
B. Ap hybridised
C. Et hybridised
D. Fe hybridised
ANSWER: A
QUESTION NUMBER 26
The type of bond in ammonium chloride is…?
A. Covalent bonds and an ionic bond
B. Polar Covalent
C. None of the above
D. NaCl
ANSWER: B
QUESTION NUMBER 27
across the period atomic radius …..?
A. Decreases
B. Increases
C. Moves About
D. Stays Stationer
ANSWER: A
QUESTION NUMBER 28
The law of octaves was proposed by…?
A. John Alexander Reina Newlands
B. Mr Obini
C. Nail Newland
D. Emmy
ANSWER: A
